Is Sodium Hydroxide Stronger Than Potassium Hydroxide
Aug 15 2020 Strong bases. Strong bases are commonly though not exclusively formed from the hydroxides of alkali metals and alkaline earth metals.
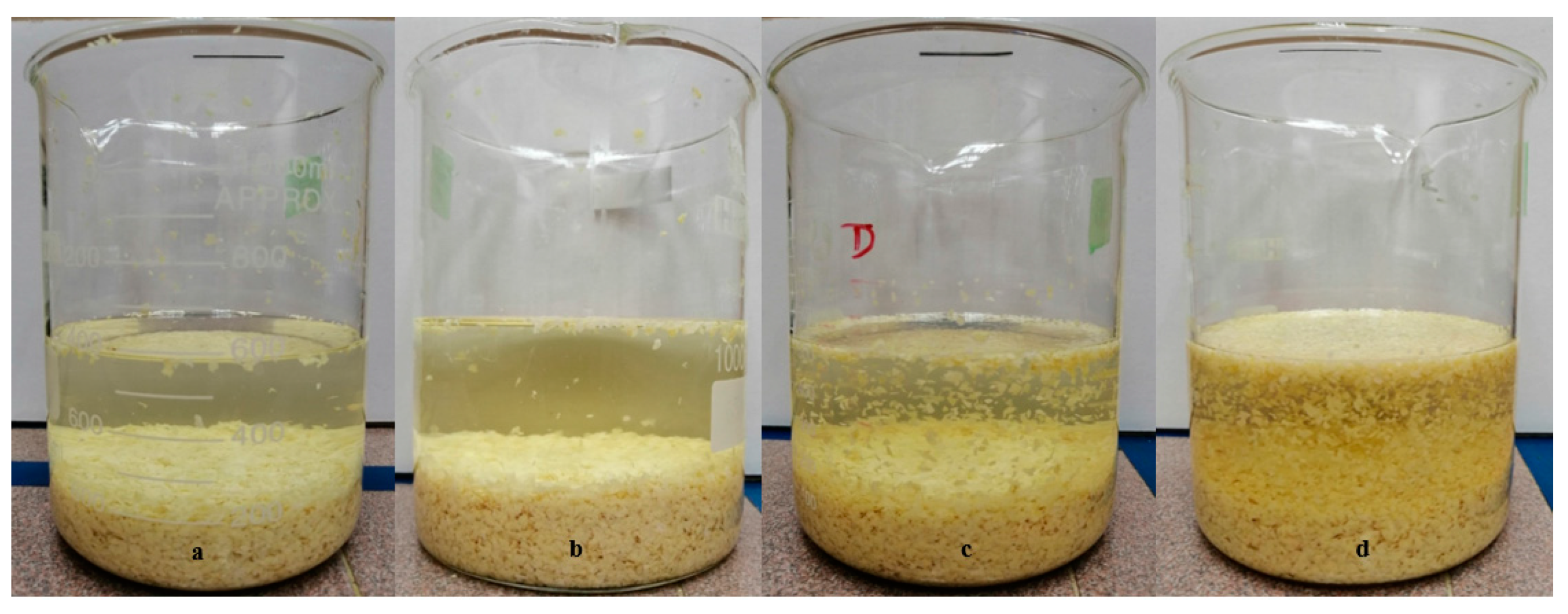 Applied Sciences Free Full Text Sodium Hydroxide Treatment Of Waste Rubber Crumb And Its Effects On Properties Of Unsaturated Polyester Composites Html
Applied Sciences Free Full Text Sodium Hydroxide Treatment Of Waste Rubber Crumb And Its Effects On Properties Of Unsaturated Polyester Composites Html
About 121 g of KOH is soluble in 100 ml of water compared to 100 g of NaOH in 100 ml of water.

Is sodium hydroxide stronger than potassium hydroxide. They are both a white strong alkaline corrosive solid or powder. Mathematically we use 14 times as much KOH as NaOH. Hence NaOH which gives OH ions easily then KOH is stronger.
Aug 03 2019 Made From. They provide examples of bases that do not contain a hydroxide ion and are therefore strong Lewis andor Bronsted-Lowry bases but not Arrhenius bases. NaOH Na OH and similarly in water the acid hydrogen chloride forms hydronium and chloride ions.
This contributes to their vastly different atomic weights of 39997 gmol and 56106 gmol respectively. Sodium is less electronegative than Potassium so NaOH is more willing to release the hydroxy group and is the stronger base. Sometimes NaOH is preferred because a smaller quantity as compared to.
The metal in potassium hydroxide is heavier than the metal in sodium hydroxide. Sep 07 2012 Sodium Hydroxide NaOH and Potassium Hydroxide KOH are almost interchangeable. Each mole of sodium hydroxide dissolves to give a mole of hydroxide ions in solution.
This weight difference reflects the difference in the number of protons in each metal. KOH is a heavier molecule than NaOH and since we weigh our catalyst we use more KOH than we use of NaOH. They have no ability to attract or donate protons H.
OH- is a strong base because on meeting any available H it will form water. Gamma butyrolactone GBL and Sodium Hydroxide or Potassium Hydroxide basically it is degreasing solvent or floor stripper mixed with drain cleaner. The word that solves this crossword puzzle is 3 letters long and begins with L.
Jan 13 2020 Likewise the metal in sodium hydroxide is lighter than that of potassium hydroxide. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. When GBL or BD or products.
They have no ability to attract or donate protons H. But their differences actually start all the way down at the molecular level. You can think of the compound as being 100 split up into metal ions and hydroxide ions in solution.
Sodium is less electronegative than Potassium so NaOH is more willing to release the hydroxy group and is the stronger base. The confusion stems from the term lye as its applied to both. Soaps made using sodium hydroxide are harder often bar soaps whereas potassium hydroxide based soaps are more water-soluble and the results are often soft soaps or liquidgel soaps.
HCl H 2 O H 3 O Cl When the two solutions are mixed the H. They are the most chemically similar of the hydroxides. Contact with even dilute solutions will also cause skin irritation and injury the severity of which will depend on the duration of contact.
One other difference is that you tend to get more lather from the potassium hydroxide than the sodium hydroxideSodium is less electronegative than Potassium so NaOH is more willing to release the hydroxy group and is the stronger base. Bases react with acids to neutralize each other at a fast rate both in water and in alcohol. Potassium hydroxide is somewhat more corrosive than sodium hydroxide.
Manufacturers usually prefer sodium hydroxide over potassium hydroxide because it is cheaper. The reaction of potassium hydroxide is less exothermic than the reaction of sodium hydroxide with water. Nov 09 2018 Best Answer for Strong Solution Of Sodium Or Potassium Hydroxide Crossword Clue.
Sodium Hydroxide is more commonly known as lye or caustic soda where Potassium Hydroxide is known as potash. The main difference between Potassium hydroxide and Sodium Hydroxide is that Potassium hydroxide has a potassium cation and a -OH anion whereas Sodium Hydroxide has a sodium cation and OH anion. Apr 06 2015 Potassium hydroxide KOH is more soluble in water than Sodium hydroxide NaOH.
Potassium has an atomic weight of 3910 and sodium has an atomic weight of 2299. Both are hydroxides of alkali metals. Reactivity with water.
Superbases are stronger than hydroxide ions and cannot be kept in water. Jan 23 2018 Potassium hydroxide and sodium hydroxide are strong bases. Eye exposure to concentrated sodium hydroxide or potassium hydroxide solutions can cause severe eye damage and possibly blindness.
When dissolved in water the strong base sodium hydroxide ionizes into hydroxide and sodium ions.
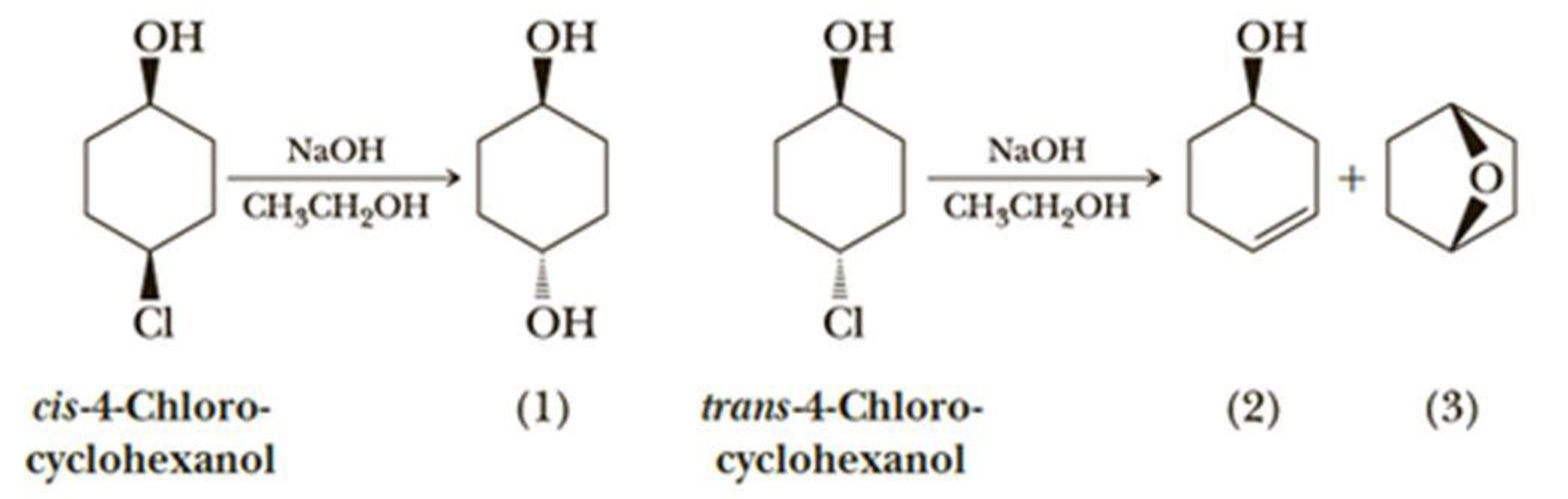 When Cis 4 Chlorocyclohexanol Is Treated With Sodium Hydroxide In Ethanol It Gives Mainly The Substitution Product Trans 1 4 Cyclohexanediol 1 Under The Same Reaction Conditions Trans 4 Chlorocyclohexanol Gives 3 Cyclohexenol 2 And The Bicyclic
When Cis 4 Chlorocyclohexanol Is Treated With Sodium Hydroxide In Ethanol It Gives Mainly The Substitution Product Trans 1 4 Cyclohexanediol 1 Under The Same Reaction Conditions Trans 4 Chlorocyclohexanol Gives 3 Cyclohexenol 2 And The Bicyclic
 Pin By Perfect Atom On Tell Me Why Do You Know What Sodium Hydroxide Calcium
Pin By Perfect Atom On Tell Me Why Do You Know What Sodium Hydroxide Calcium
 Solved Question 10 Status Not Yet Answered Points Possib Chegg Com
Solved Question 10 Status Not Yet Answered Points Possib Chegg Com
 Sodium Hydroxide Vs Potassium Hydroxide Reaction With Aluminum Youtube
Sodium Hydroxide Vs Potassium Hydroxide Reaction With Aluminum Youtube
 Using Expired Lye In Cold Process Soapmaking Soap Queen
Using Expired Lye In Cold Process Soapmaking Soap Queen
 What S The Difference Between Sodium Hydroxide And Potassium Hydroxide For Soapmaking Rusticwise
What S The Difference Between Sodium Hydroxide And Potassium Hydroxide For Soapmaking Rusticwise
 How To Make Sodium Hydroxide Lye From Baking Soda Youtube
How To Make Sodium Hydroxide Lye From Baking Soda Youtube
 The Difference Between Sodium And Potassium Hydroxide Https Www Youtube Com Watch V 3umpstivej8 List Uu518jem4 X4iwhukizfqw Potassium Sodium Sodium Hydroxide
The Difference Between Sodium And Potassium Hydroxide Https Www Youtube Com Watch V 3umpstivej8 List Uu518jem4 X4iwhukizfqw Potassium Sodium Sodium Hydroxide
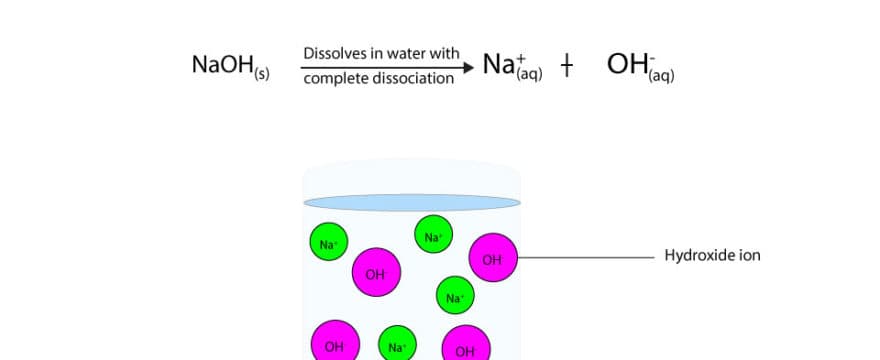 Why S Sodium Hydroxide A Strong Base While Ammonia A Weak Base
Why S Sodium Hydroxide A Strong Base While Ammonia A Weak Base
 Using Expired Lye In Cold Process Soapmaking Soap Queen
Using Expired Lye In Cold Process Soapmaking Soap Queen
 Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
 Naoh Hcl Sodium Hydroxide Hydrochloric Acid Net Ionic Equation Youtube
Naoh Hcl Sodium Hydroxide Hydrochloric Acid Net Ionic Equation Youtube
Base Sodium Hydroxide Naoh In Water Strong Electrolyte
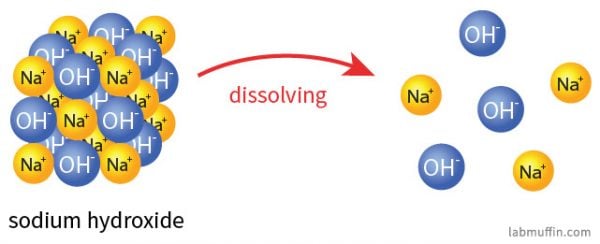 Is Sodium Hydroxide Safe In Beauty Products Lab Muffin Beauty Science
Is Sodium Hydroxide Safe In Beauty Products Lab Muffin Beauty Science
 Side By Side Comparison Potassium Hydroxide And Sodium Hydroxide Similarities Differences And Use Cases
Side By Side Comparison Potassium Hydroxide And Sodium Hydroxide Similarities Differences And Use Cases
 Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table
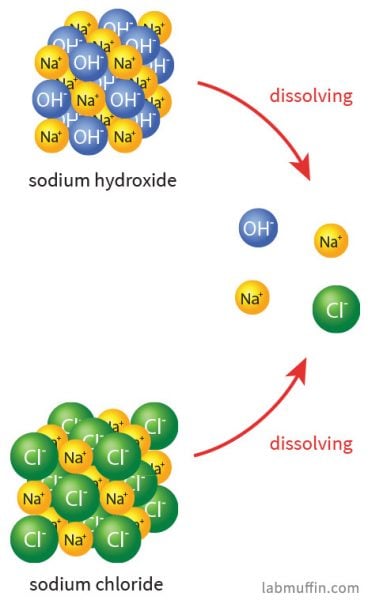 Is Sodium Hydroxide Safe In Beauty Products Lab Muffin Beauty Science
Is Sodium Hydroxide Safe In Beauty Products Lab Muffin Beauty Science
 Strength Of Bases Chemistry Master
Strength Of Bases Chemistry Master
